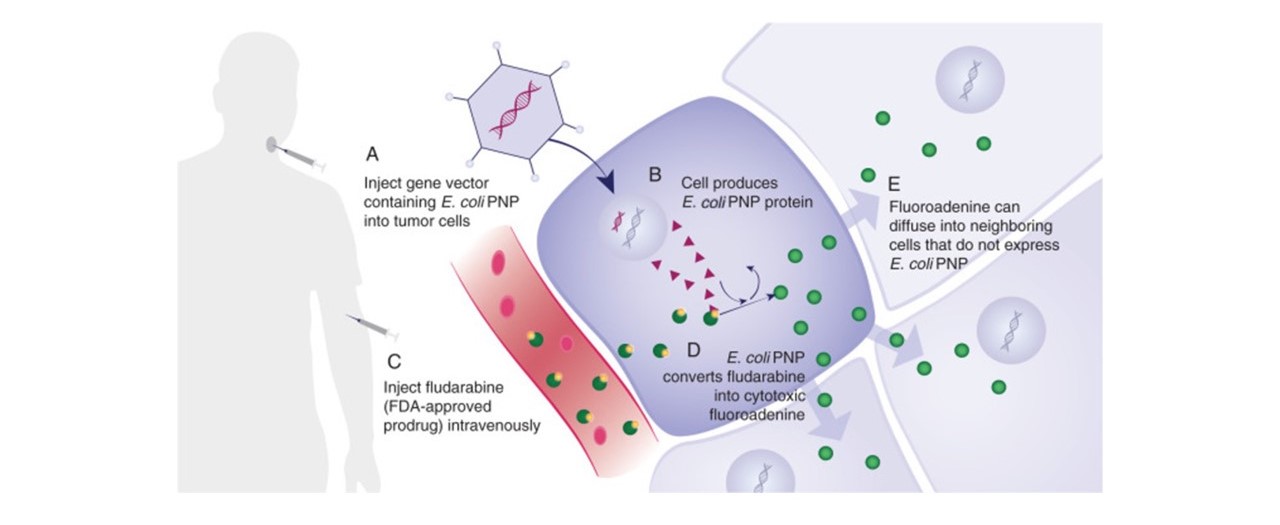
GEDEPTIN TECHNOLOGY OVERVIEW
A Phase 1/2 trial (NCT03754933), evaluating the safety and efficacy of repeat cycles of Gedeptin therapy in patients with recurrent head and neck squamous cell carcinoma (HNSCC), with tumor(s) accessible for injection and no curable treatment options recently completed enrollment at the Stanford University Cancer Institute, the Emory University Winship Cancer Institute, and the Thomas Jefferson University Sidney Kimmel Cancer Center.
The trial design involved repeat administration using Gedeptin followed by systemic fludarabine (prodrug). Expansion towards a larger, Phase 2 patient trial is anticipated. The FDA has granted Gedeptin orphan drug status for the intra-tumoral treatment of anatomically accessible oral and pharyngeal cancers, including cancers of the lip, tongue, gum, floor of mouth, salivary gland and other oral cavities. Also, the initial Phase 1/2 clinical study was funded by the FDA pursuant to its Orphan Products Clinical Trials Grants Program.